What is surface tension
Surface tension is defined as the work, dW, required to expand a surface by dA. It is, thus, a direct measure of the energy needed to form a new surface of unit area, i.e. surface energy. Surface tension is a characteristic of any surface or interface, however, it is only directly measurable for liquids. Surface tension results from direction dependent forces of molecules residing at or close to the interface. Surface tension is generally expressed in N/m (SI unit).
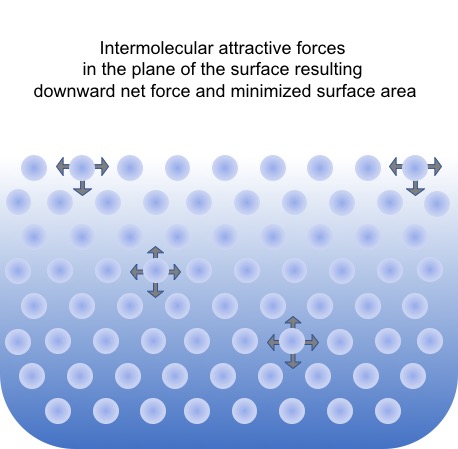
Typically, polar solvents have higher surface tension than their non-polar counterparts. For example, the high surface tension of water (72.8 mN/m at 20°C) is due to the strong intermolecular hydrogen bonding (electrostatic interaction between the partially positive hydrogens and the partially negative oxygen atom of a neighboring water molecule). The weaker the intermolecular forces are, the lower the surface tension is (e.g. octane has a surface tension of 21.6 mN/m). Usually, the term “interfacial tension” is used for the tension between two liquids, while “surface tension” is more general and usually referring to an gas-liquid interface.
When is surface tension relevant
Surface tension influences properties in a system. The smaller the dimensions of a system are, the more critical surface tension becomes. Examples include emulsions, mists and foams, nucleation & phase formation. Surface tension is relevant at larger scales as well, particularly in wetting, adhesion, and meniscus shape. When the dimensions are in the micro- to millimetre range, dynamics are given by a balance of viscosity, density, and surface tension. Additionally, surface tension is an easily measured parameter for quality control.
Surfactants
Surfactants are molecules that enrich at surfaces or interfaces, i.e. adsorb at the interface. In general, surfactants contain hydrophilic and hydrophobic moieties, and hence in aqueous solutions water contact with the hydrophobic parts can be avoided by arranging at the surface with the hydrophobic parts towards the air. Surfactant adsorption lowers the surface tension. This phenomenon is frequently used to improve wetting. Click the image below to read more on adsorption and surface tension.
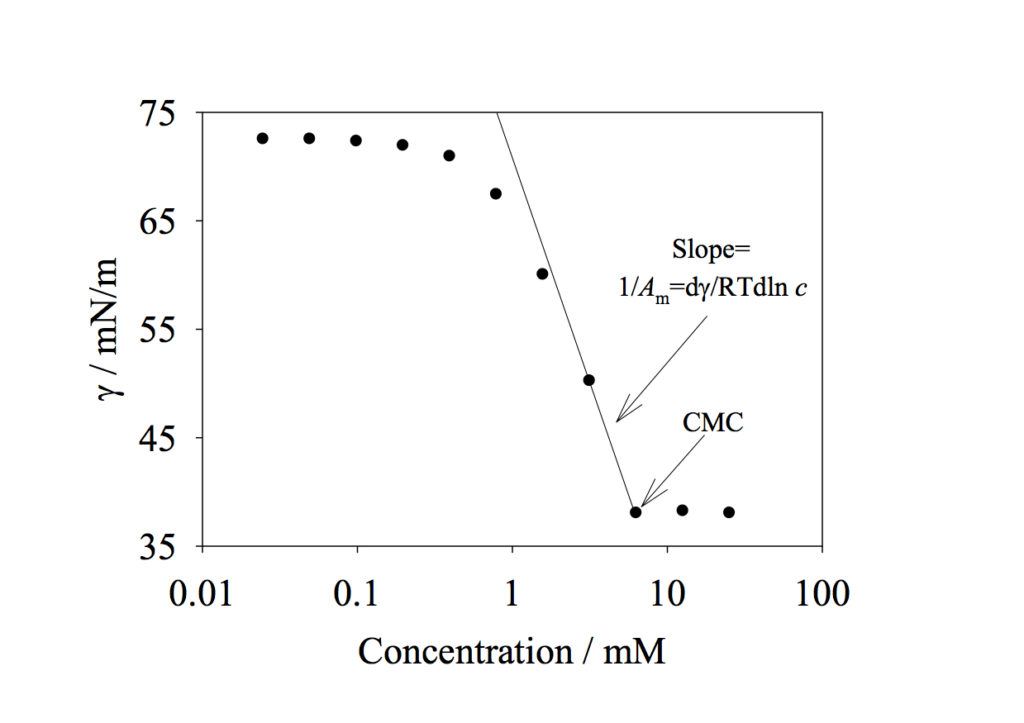
Surfactants display another important property, namely self-assembly into micelles. Micellation resembles phase formation, in that many surfactants form one micelle. Thus, micellation occurs over a fairly narrow concentration range (critical micelle concentration, CMC), and further addition of surfactant increases the micelle concentration rather than the concentration of surfactant monomers. Consequently, surface tension decreases with increasing total surfactant concentration up to CMC, and then remains constant. Surface tension measurement is thus a convenient way to study micellation.
Measuring surface tension
Several methods have been developed to measure surface tension. In optical techniques, the shape of a droplet or meniscus is analysed usually using a computer. The shape assumes mechanical equilibrium between the forces acting on the liquid, i.e. surface tension and gravitational forces. To obtain accurate results, the optical setup needs to be well calibrated, and furthermore the outline of the droplet determined exactly.
In bubble pressure tensiometry, one uses the fact that the pressure inside a bubble is higher than outside the droplet due surface tension. The surface tension can be calculated from the pressure difference if the radius and droplet shapes are known. With a few simple assumptions, one can build easy to use and robust instrumentation which is well suited for coarse measurement of surface tension just by immersing a tube into the solution. Stalagometry is a variation of the bubble pressure technique. Instead of measuring the pressure difference, the fact that the flow rate of a liquid through a capillary is determined by the gravitational forces opposed by viscous force and counter pressure from the droplet at the tip of the capillary.
The oldest technique to measure surface tension is to measure the force exerted by a liquid on a probe. Force tensiometry techniques are fairly exact provided that the contact angle at the probe is known. By choosing a well wetted material, and assuming that the contact angle is zero. For aqueous solutions this implicates the use of a hydrophilic material. The most common material is platinum. Experiments conducted under controlled and inert atmosphere have shown that water indeed completely wets platinum. However, many compounds found in the ambient air adsorb strongly on platinum, the effect is observable within minutes of flaming. Thus, the probe should be immersed in the measured liquid promptly after cleaning.
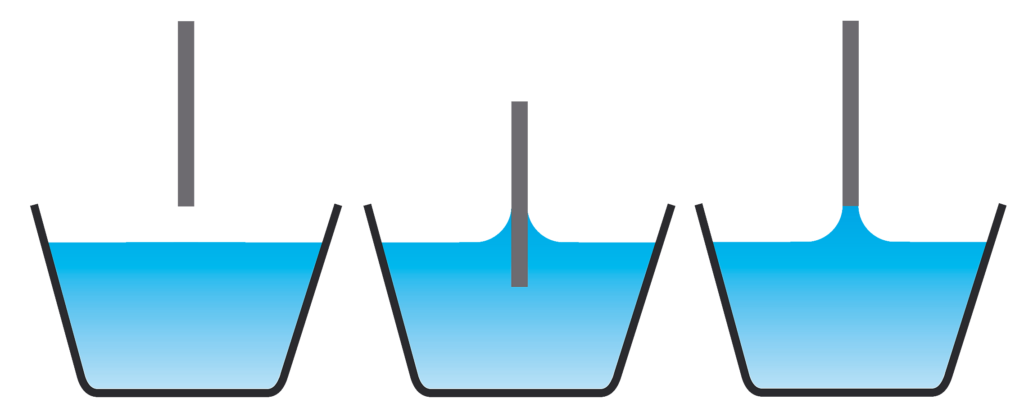
Kibron’s Dyneprobe is based on a hydrophilic oxide. When flame cleaned using a hot flame, the oxide layer is activated into a very hydrophilic state. It is more stable towards airborne contaminants than Platinum, but does passivate with time. Therefore, proper flame cleaning procedures cannot be avoided. There are two sub-techniques of force tensiometry, namely the Wilhelmy and DuNouy methods. In the Wilhelmy method, the probe is held still in the interface, preferentially with the lower end level with the interface (at far distance). At this point there is no need for buoyancy corrections. Both so called Wilhelmy plates and rod shaped probes can be used to make the measurement.
In the DuNouy (maximum pull force) method the surface tension is recorded as the probe is slowly withdrawn from the liquid. The probe is usually a DuNouy ring or a vertical rod. The movement of the probe advantageously provides a receding contact angle, which tends to be smaller than the resting contact angle. The drawback of the technique is the need to account for the negative buoyancy term corresponding to the point of maximum force where the probe is completely above the surface. For thin rod probes the buoyancy term is relatively small and easy to correct for, while for rings the buoyancy term is significant and its calculation complicated by the cross-sectional shape of the ring.