Critical Micelle Concentration
The defining characteristic of amphiphilic molecules or more commonly surfactants (surface active compound) is their ability to lower surface tension by partitioning into the interface. This accumulation of a surface active compound in the air-water interface lowers the surface tension. This phenomenon is well described by classical thermodynamics, more specifically, by the Gibbs adsorption isotherm.
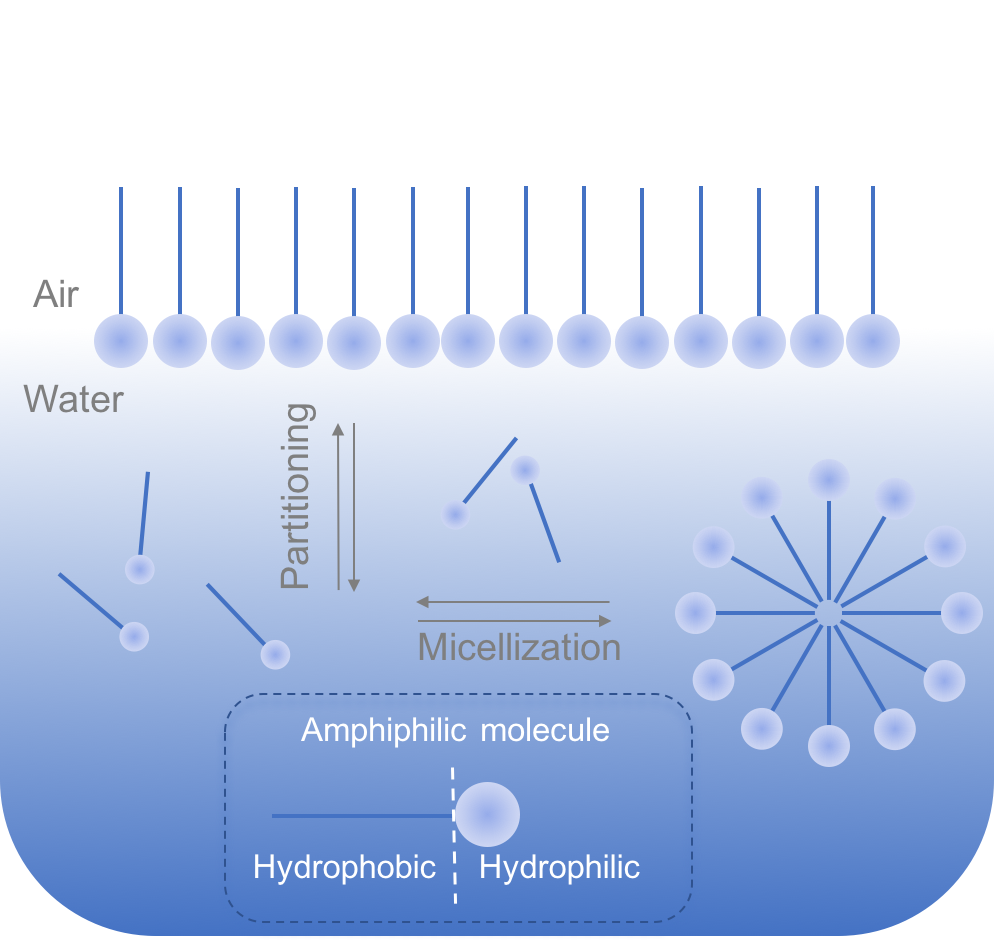
(amphiphilic molecule) at a surface, free surfactant, and in micelles.
Surfactants possess another important property, namely self-assembly into micelles in the solution. Micelles are structure where the surfactants minimize unfavourable interaction with the solvent, for example in aqueous solution hydrophobic surfactants chains assemble in the middle of the micelles.
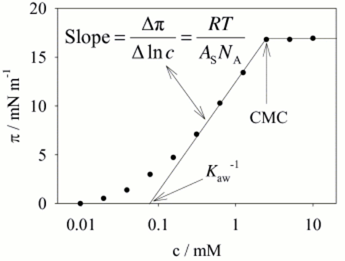
from surface tension vs. concentration data.
Since many surfactants take part in a micelles the phenomenon is similar to phase formation, with the micelle formation occurring over a fairly narrow concentration range of free surfactant. Thus, the critical micelle concentration (CMC) or solubility limit results in a sharp transition above which the concentration of the free surfactant molecules remain constant, resulting in a plateau in the surface tension / pressure. Surface tension measurements is one of the most efficient techniques to determine CMC. However, independently of the experimental technique chosen, CMC determination requires analysis at several concentrations, usually amounting to more than 10 points. The Critical Micelle Concentration is then determined from a plot of Surface Tension vs. the logarithm of the concentration. The Delta-8 is designed for the task.
Micelles are industrially interesting structures. They can serve for example as surfactant reservoirs, or nano-sized containers for chemicals, for example hydrophobic species can be solubilized in the hydrophobic environment in the micelle core. Applications range from emulsion polymerization to drug carrier vehicles.
Kibron Delta-8 is a unique high throughput surface tension plate reader, for e.g. HTS screening of critical micelle concentration (CMC) values of surface active materials. It is a fully automated platform capable of taking 96 surface tension measurements in 2-10 minutes which is about 100 times faster than conventional instruments. The instrument uses wire probes that are inexpensive and robust compared to Wilhelmy plates and Du Nuoy rings. The automated cleaning of the probes in the probe cleaning unit prior to each measurement cycle reduces manual maintenance considerably. The instrument is well suited for expensive materials due to the low volume required
The Delta-8 uses standard size detection plates and conventional dispensing systems. Each well (volume 50 μl) can be charged with aliquots of solution of different concentration. Variation of assay conditions (eg. pH, salt concetration) or testing of binary combinations of different excipients can be done rapidly. Below is an example assay variating surfactant composition in a 96-wel plate with polymer and surfactant. The data was produced in just one hour with the Kibron Delta-8.
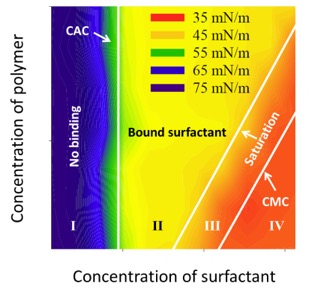
polystyrenesulfonate and cetylpyridinium concentration
The Delta-8 system is easily integrated into conventional laboratory robotics using our dedicated communication interface. The Delta-8 Manager software is easy to use and encourages proper design of experiments while providing elementary analysis of isotherms.
